About Our Study
Study Participants
People who have worked in a long-term care setting in the United States in the past two years are invited to join the study. Long-term care settings include: nursing homes, skilled nursing facilities, assisted living facilities, home health care, hospice care, and retirement communities.
Study Design
Study participants will be randomly assigned to one of our three trial arms. We will review the results to determine if there are differences in the primary and secondary outcomes between the three trial arms.
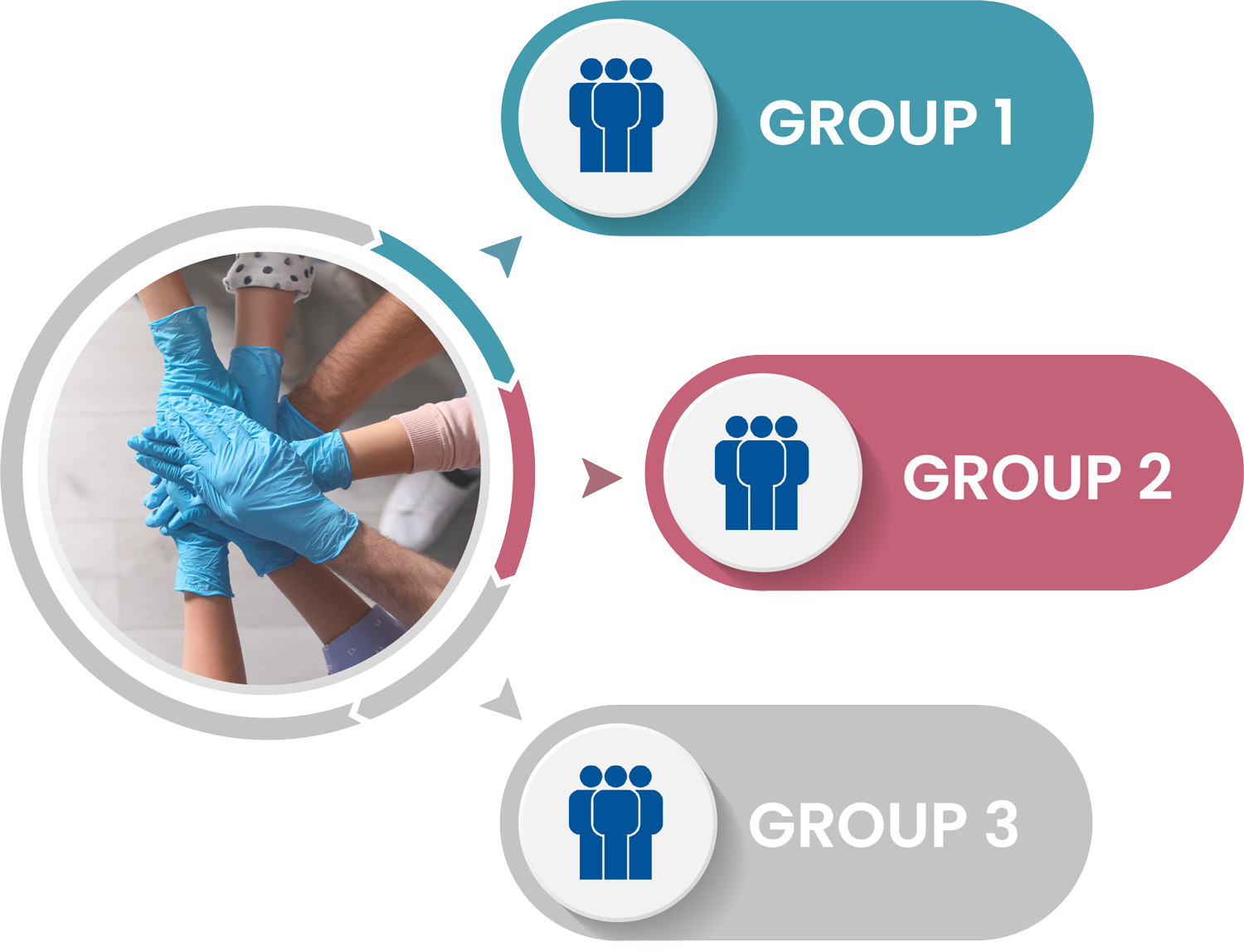
Trial Arms
We will compare two different online interventions to usual information available online. The interventions will give information and provide support to long-term care workers to help them feel more confident about the COVID-19 vaccines.
Primary Outcome
We will compare long-term care worker confidence in the vaccines across the three trial arms. We will also measure other things to better understand how the trial interventions might influence people.
Sustainability Period Stays At 8 Months
During the active trial phase, we will also study the best ways to implement and deliver the interventions. Our findings will support adapting and offering the interventions to other long-term care workers as part of an 8-month sustainability period. These findings will also inform future efforts to scale up the interventions.
Timeline
Trial Set Up and Intervention Development
August 2021 – February 2022
Intervention Delivery and Follow Up
February 2022 – April 2023
Study of Intervention Sustainability Requirements
December 2021 – January 2024
Sustainability Period
October 2023 – May 2024

Results and Dissemination
January 2024 – July 2024
